Dosing for ZERBAXA® (ceftolozane and tazobactam)
Learn about:
Dosing and administration
Dosage for adult patients with hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia (HABP/VABP)a,b
The recommended dose of ZERBAXA in adult patients 18 years and older with HABP/VABP and creatinine clearance (CrCl) greater than 50 mL/min is 3 grams (two 1.5 g vials) over 1-hour period every 8 hours for 8 to 14 days.
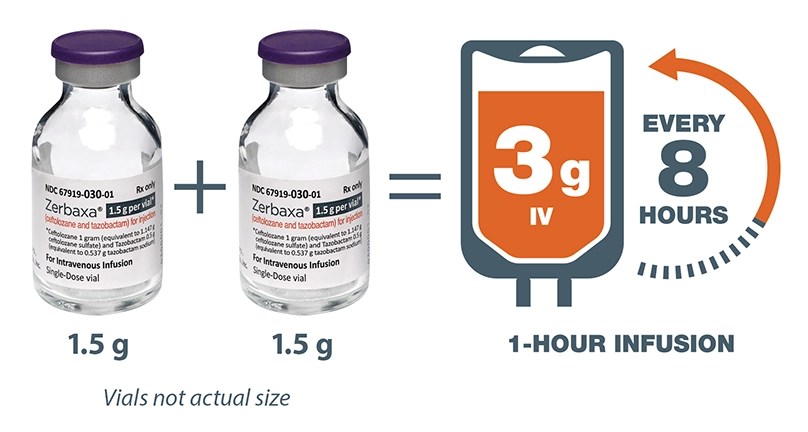

Renal dosing adjustments for adult patients with HABP/VABP per estimated CrCl (mL/min)a,b
- 30 to 50 CrCl (mL/min) 1.5 g (1 g and 0.5 g) intravenously every 8 hours
- 15 to 29 CrCl (mL/min) 750 mg (500 mg and 250 mg) intravenously every 8 hours
- For patients with end-stage renal disease on hemodialysis: a single loading dose of 2.25 g (1.5 g and 0.75 g) followed by a 450-mg (300 mg and 150 mg) maintenance dose administered intravenously every 8 hours for the remainder of the treatment period (on hemodialysis days, administer the dose at the earliest possible time following completion of dialysis).
aCreatinine clearance (CrCl) estimated using Cockcroft-Gault formula.
bAll doses of ZERBAXA are administered over 1 hour.
3-g dose selected based on Phase 1 PK/PD study
- ZERBAXA at 3-g IV dose achieved target concentration in the epithelial lining fluid (ELF) over 100% of the dosing interval.
PK/PD study design
- Following 1-hour intravenous infusions of ZERBAXA 3 g (ceftolozane 2 g and tazobactam 1 g) or adjusted based on renal function every 8 hours in ventilated adult patients with confirmed or suspected pneumonia (N=22), mean pulmonary epithelial lining fluid-to-free plasma AUC ratios of ceftolozane and tazobactam were approximately 50% and 62%, respectively, and are similar to those in healthy subjects (approximately 61% and 63%, respectively) receiving ZERBAXA 1.5 g (ceftolozane 1 g and tazobactam 0.5 g)
- Minimum ceftolozane and tazobactam epithelial lung lining fluid concentrations in ventilated subjects at the end of the dosing interval were 8.2 mcg/mL and 1.0 mcg/mL, respectively
Dosage for adult patients (18 years and older) with cIAI and cUTI with CrCl > 50 mL/mina,b
Complicated intra-abdominal infectionsc
| Dose (g) | 1.5 |
| Frequency | Every 8 hours |
| Infusion time | 1 hour |
| Duration of treatment | 4 to 14 days |
a Creatinine clearance (CrCl) estimated using Cockcroft-Gault formula.
b All doses of ZERBAXA are administered over 1 hour.
c Used in conjunction with metronidazole 500 mg intravenously every 8 hours.
Complicated urinary tract infections, including pyelonephritis
| Dose (g) | 1.5 |
| Frequency | Every 8 hours |
| Infusion time | 1 hour |
| Duration of treatment | 7 days |
Dosage adjustments in adult patients (18 years and older) with renal impairment
Dose adjustment is required for patients with CrCl 50 mL/min or less. All doses of ZERBAXA are administered over 1 hour. For patients with changing renal function, monitor CrCl at least daily and adjust the dosage of ZERBAXA accordingly.
Renal dosing adjustments for adult patients (18 years and older) with cIAI and cUTI with CrCl 50 mL/min or lessa, b
- 30 to 50 CrCl (mL/min) 750 mg (500 mg and 250 mg) intravenously every 8 hours
- 15 to 29 CrCl (mL/min) 375 mg (250 mg and 125 mg) intravenously every 8 hours
- For patients with end-stage renal disease (ESRD) on hemodialysis (HD): a single loading dose of 750 mg (500 mg and 250 mg) followed by a 150 mg (100 mg and 50 mg) maintenance dose administered every 8 hours for the remainder of the treatment period (on hemodialysis days, administer the dose at the earliest possible time following completion of dialysis).
Dosage for pediatric patients (birth to less than 18 years of age) with eGFRd greater than 50 mL/min/1.73 m2
Complicated intra-abdominal infectionse
| Dose (g) | 30 mg/kg up to a maximum dose of 1.5 gf |
| Frequency | Every 8 hours |
| Infusion time | 1 hour |
| Duration of treatment | 5 to 14 days |
Complicated urinary tract infections, including pyelonephritis
| Dose (g) | 30 mg/kg up to a maximum dose of 1.5 gf |
| Frequency | Every 8 hours |
| Infusion time | 1 hour |
| Duration of treatment | 7 to 14 days |
dEstimated GFR using an age-appropriate equation for use in the pediatric population.
eUsed in conjunction with metronidazole.
fPediatric patients weighing greater than 50 kg should not exceed a maximum dose of 1.5 g.
Dosage adjustments in pediatric patients (birth to less than 18 years of age) with renal impairment
Dosage adjustment of ZERBAXA in pediatric patients (birth to less than 18 years of age) with eGFR 50 mL/min/1.73 m2 or less has not been determined.
ZERBAXA is not recommended in pediatric patients who have an eGFR 50 mL/ min/1.73m2 or less.
Dosing, administration, and billing guide

Download the dosing and billing guide for necessary coding, coverage, reimbursement, and billing information for ZERBAXA.
