STELLAR: A 24-week study of adults with PAH (WHO Group 1, FC II or III) on background therapya
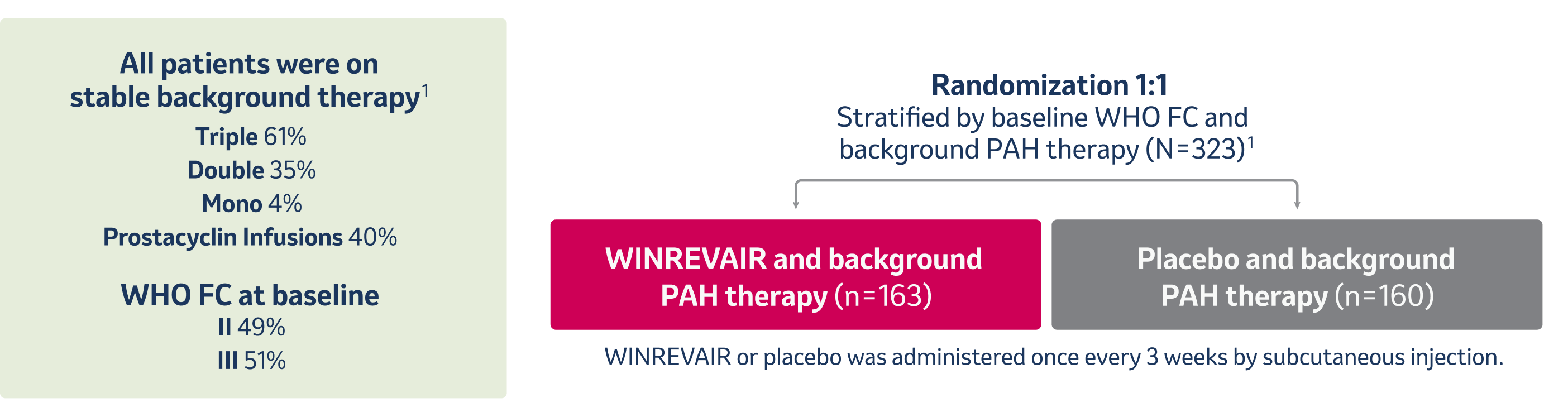
All patients were receiving stable background therapy for PAH for at least 90 days before enrollment and continued receiving background therapy throughout the study.1
aSTELLAR was a 24-week, global, double-bind, placebo-controlled, multicenter, parallel-group, phase 3 study in adults with PAH.1
The STELLAR study excluded patients with human immunodeficiency virus–associated PAH, PAH associated with portal hypertension, schistosomiasis-associated PAH, and pulmonary veno-occlusive disease.
STELLAR study endpoints
Primary endpoint: Change from baseline in 6-minute walk distance (6MWD) at week 24
Selected secondary endpoints: Change from baseline at week 24 in1 :
- Pulmonary vascular resistance (PVR)
- N-terminal pro-B-type natriuretic peptide (NT-proBNP)
- Proportion of patients who improved WHO FC
- Time to death or the first occurrence of a PAH clinical worsening event (TTDCW)ᵇ
bThese outcomes were captured until the last patient completed the week 24 visit (median duration of exposure 33.6 weeks).
Selected baseline characteristics
Mean time from PAH diagnosis to screening
- 8.8 years
Median age
- 48 years (range: 18 to 82 years)
Median weight
- 68 kg (range: 38 to 141 kg)
Most common PAH etiologies
- Idiopathic PAH: 59%
- Heritable PAH: 18%
- PAH associated with connective tissue diseases (CTD): 15%
Sex
- Female: 79%
Race and ethnicity
- White: 89%
- Black/African American: 2%
- Asian: 2%
- American Indian or Alaska Native: 0.3%
- Native Hawaiian or other Pacific Islander: 0.3%
- Missing/other races: 6%
Reference:
1. Hoeper MM, Badesch DB, Ghofrani HA, et al; STELLAR Trial Investigators. Phase 3 trial of sotatercept for treatment of pulmonary arterial hypertension. N Engl J Med. 2023;388(16):1478-1490.
