Clinical efficacy of ZERBAXA® (ceftolozane and tazobactam) against cUTIs
Learn about:
Complicated urinary tract infections (cUTIs) in adult patients, including pyelonephritis
A total of 1068 adults hospitalized with cUTI (including pyelonephritis) were randomized and received study medications in a multinational, double-blind study comparing ZERBAXA 1.5 g (ceftolozane 1 g and tazobactam 0.5 g) intravenously every 8 hours to levofloxacin (750 mg intravenously once daily) for 7 days of therapy.
The primary efficacy endpoint was defined as complete resolution or marked improvement of the clinical symptoms and microbiological eradication (all uropathogens found at baseline at ≥105 were reduced to <104 CFU/mL) at the test-of-cure (TOC) visit 7 (± 2) days after the last dose of study drug.
The primary efficacy analysis population was the microbiologically modified intent-to-treat (mMITT) population, which included all patients who received study medication and had at least 1 baseline uropathogen. The key secondary efficacy endpoint was the composite microbiological and clinical cure response at the TOC visit in the microbiologically evaluable (ME) population, which included protocol-adherent mMITT patients with a urine culture at the TOC visit.
The mMITT population consisted of 800 patients with cUTI, including 656 (82%) with pyelonephritis. The median age was 50.5 years and 74% were female. Concomitant bacteremia was identified in 62 (7.8%) patients at baseline; 608 (76%) patients were enrolled in Eastern Europe and 14 (1.8%) patients were enrolled in the United States.
ZERBAXA demonstrated efficacy with regard to the composite endpoint of microbiological and clinical cure at the TOC visit in both the mMITT and ME populations (table below). Composite microbiological and clinical cure rates at the TOC visit by pathogen in the mMITT population are presented in the table below.
In the mMITT population, the composite cure rate in ZERBAXA-treated patients with concurrent bacteremia at baseline was 23/29 (79.3%).
Although a statistically significant difference was observed in the ZERBAXA arm compared to the levofloxacin arm with respect to the primary endpoint, it was likely attributable to the 212/800 (26.5%) patients with baseline organisms non-susceptible to levofloxacin. Among patients infected with a levofloxacin-susceptible organism at baseline, the response rates were similar (table below).
Composite microbiological and clinical cure rates in a Phase 3 trial of complicated urinary tract infections in adult patients

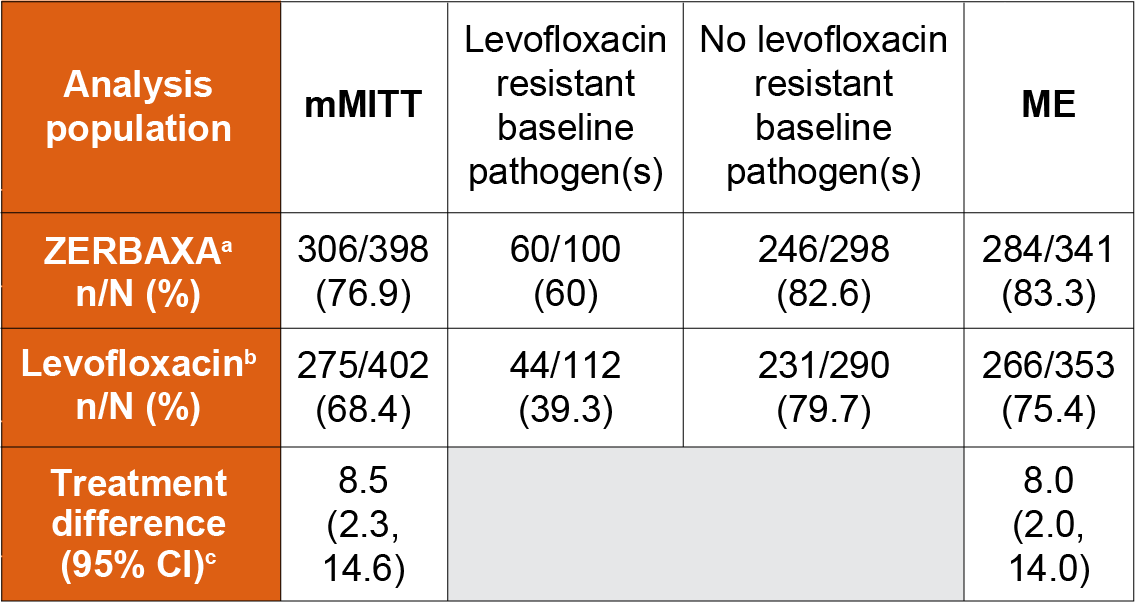
aZERBAXA 1.5 g intravenously every 8 hours.
b750 mg intravenously once daily.
cThe 95% confidence interval was based on the stratified Newcombe method.
Composite microbiological and clinical cure rates in a Phase 3 trial of complicated urinary tract infections, in subgroups defined by baseline pathogen (mMITT population)
| Pathogen | ZERBAXA n/N (%) | Levofloxacin n/N (%) |
|---|---|---|
| Escherichia coli | 247/305 (81) | 228/324 (70.4) |
| Klebsiella pneumoniae | 22/33 (66.7) | 12/25 (48) |
| Proteus mirabilis | 11/12 (91.7) | 6/12 (50) |
| Pseudomonas aeruginosa | 6/8 (75) | 7/15 (46.7) |
In a subset of the E. coli and K. pneumoniae isolates from both arms of the cUTI Phase 3 trial that met pre-specified criteria for beta-lactam susceptibility, genotypic testing identified certain ESBL groups (e.g., TEM, SHV, CTX-M, OXA) in 104/687 (15%). Cure rates in this subset were similar to the overall trial results. In vitro susceptibility testing showed that some of these isolates were susceptible to ZERBAXA (MIC ≤2 mcg/mL), while some others were not susceptible (MIC >2 mcg/mL). Isolates of a specific genotype were seen in patients who were deemed to be either successes or failures.
Complicated urinary tract infections (cUTIs) in pediatric patients, including pyelonephritis
The cUTI pediatric trial was a randomized, double-blind multi-center, active controlled trial conducted in hospitalized patients from birth to less than 18 years (NCT03230838). Eligible patients were randomized in a 3:1 ratio to IV ZERBAXA or meropenem, respectively. Patients received IV study treatment for a minimum of 3 days before an optional switch to oral step-down therapy at the discretion of the investigator to complete a total of 7 to 14 days of antibacterial therapy.
The microbiologic modified intent-to-treat (mMITT) population consisted of 95 patients (N=71 in the ZERBAXA group; N=24 in the meropenem group) who were randomized and received at least one dose of study treatment and had an eligible uropathogen isolated from a baseline urine culture.
The median age of patients was 2.7 years and 1.6 years in the ZERBAXA and meropenem groups, respectively. In the ZERBAXA group, enrollment by age group was as follows: 12 to <18 y: n=10, 6 to <12 y: n=13, 2 to <6 y: n=14, 3 months to <2 y: n=20, birth to <3 months: n=14. Patients treated with ZERBAXA were predominantly female (56%) and White (99%). Patients treated with meropenem were predominantly female (63%) and White (100%). Most patients in the mMITT population had a diagnosis of pyelonephritis (ZERBAXA: 84.5%; meropenem: 79.2%). The most common baseline qualifying gram-negative uropathogens were Escherichia coli (ZERBAXA: 74.6%; meropenem: 87.5%), Klebsiella pneumoniae (8.5%; 4.2%), and Pseudomonas aeruginosa (7.0%; 8.3%).
The primary objective of the study was to evaluate the safety and tolerability of ZERBAXA. Efficacy assessments were not powered for formal hypothesis testing of between treatment group comparisons. At the TOC visit, which occurred 7 to 14 days after the last dose of study drug, a favorable clinical response was defined as complete resolution or marked improvement in signs and symptoms of the cUTI or return to pre-infection signs and symptoms, such that no further antibiotic therapy (IV or oral) was required for the treatment of the cUTI. A favorable microbiological response at the TOC was defined as eradication (all uropathogens found at baseline at ≥105 were reduced to <104 CFU/mL) of baseline uropathogens from the urine culture. A summary of clinical and microbiologic response rates in the mMITT population at the TOC visit is presented in the table below.
Clinical and microbiological response rates in a pediatric study of complicated urinary tract infections
| mMITT population | ZERBAXA n/N (%) | Meropenem n/N (%) | Treatment difference (95% CI)d |
|---|---|---|---|
| Clinical Response Rate | 63/71 (88.7) | 23/24 (95.8) | -7.3 (-18.0, 10.1) |
| Microbiologic Response Rate | 60/71 (84.5) | 21/24 (87.5) | -3.0 (-17.1, 17.4) |
dThe Miettinen & Nurminen method stratified by age group with Cochran-Mantel-Haenszel weights was used.
