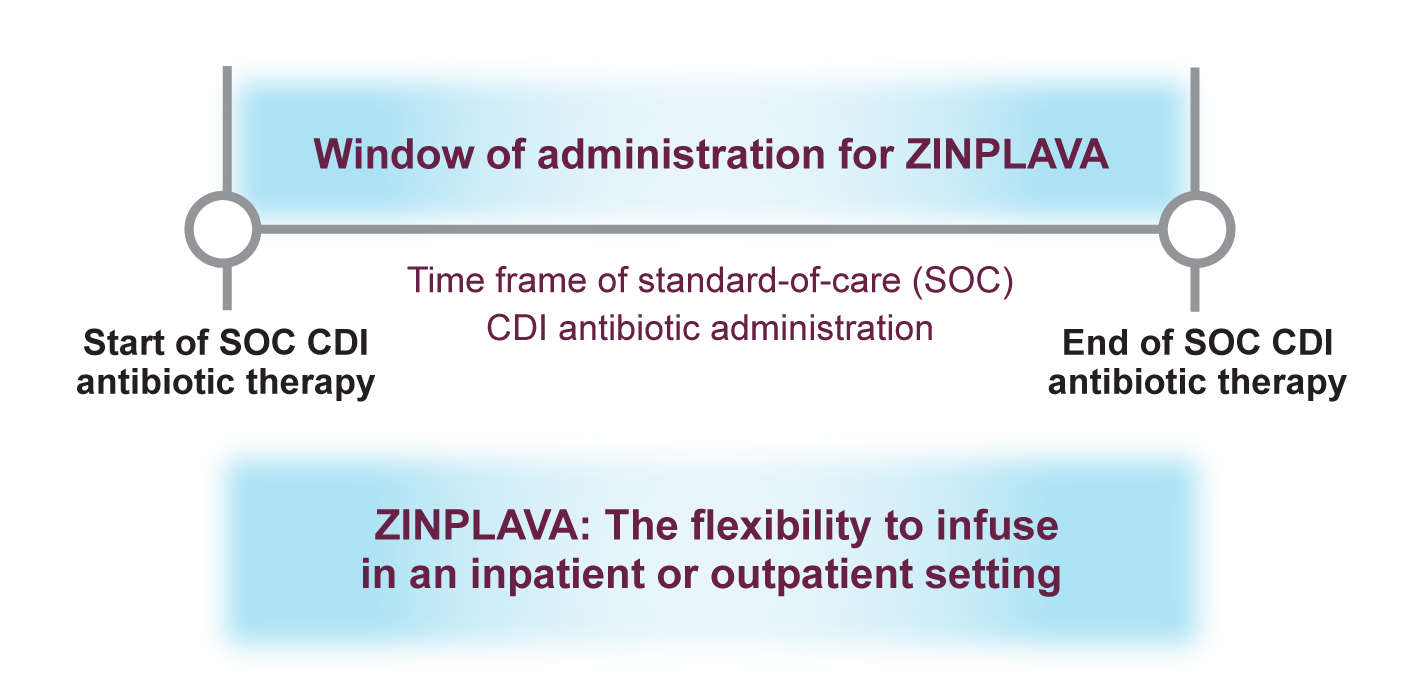
Dosing and administration
Learn About:
Appropriate patients for ZINPLAVA
Think about adding ZINPLAVA when your patients are receiving antibacterial drug treatment for CDI and have 1 or more of these risk factors:
- History of CDI in the previous 6 months
- Patients ≥65 years of age
- Patients who are immunocompromised
- Severe CDI (Zar score ≥2)
Add ZINPLAVA: Target toxin B to reduce CDI recurrence
The recommended dose of ZINPLAVA is a single 10-mg/kg dose administered as an IV infusion over 60 minutes.