Mechanism of action
ZINPLAVA is the first and only FDA-approved human monoclonal antibody to reduce Clostridioides difficile infection (CDI) recurrence in patients aged ≥18 years receiving antibacterial drug treatment of CDI who are at high risk for CDI recurrence.
Novel mechanism of action targets toxin B
ZINPLAVA is a human monoclonal antibody that binds to C. difficile toxin B and neutralizes its effects. ZINPLAVA is not an antibiotic.
In vitro, ZINPLAVA neutralized the pathogenic action of toxin B
The clinical significance of in vitro data is unknown.
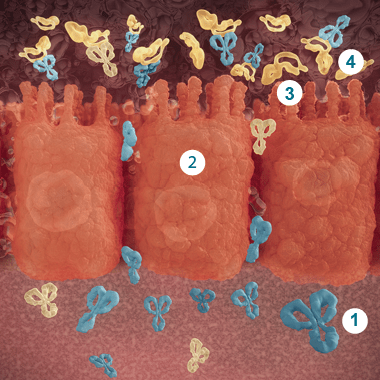
- ZINPLAVA
- Damaged gut epithelial cells
- Toxin B
- ZINPLAVA binding to toxin B
Adapted from Zhang Z et al. Infect Immun. 2015;83(1):405–416.
- ZINPLAVA is a circulating antibody with a half-life of ~19 days.
- Damage to the gut epithelium allows ZINPLAVA to move from the systemic side to the luminal side.1
- ZINPLAVA targets toxin B, helping to reduce the likelihood of CDI recurrence.1
- ZINPLAVA does not bind to C. difficile toxin A.
- In a ZINPLAVA Phase 3 clinical trial, the use of antitoxin A was not shown to reduce the likelihood of CDI recurrence.2
References
1. Zhang Z, Chen X, Hernandez LD, et al. Toxin-mediated paracellular transport of antitoxin antibodies facilitates protection against Clostridioides difficile infection. Infect Immun. 2015;83(1):405–416. doi:10.1128/IAI.02550-14
2. Wilcox MH, Gerding DN, Poxton IR, et al. Bezlotoxumab for prevention of recurrent Clostridioides difficile infection. N Engl J Med. 2017;376(4):305–317. doi:10.1056/NEJMoa1602615