Dosing for PREVYMIS® (letermovir) in CMV R+ allogeneic HSCT patients
Once-daily dosing with PREVYMIS as early as the day of transplant
- Start PREVYMIS as early as day 0 and no later than day 28 post-transplant.
- PREVYMIS can be initiated before or after engraftment.
- Following the completion of prophylaxis with PREVYMIS, monitoring for CMV reactivation in HSCT recipients is recommended.
Oral or IV dosing
The recommended dosage of PREVYMIS is 480 mg administered orally or intravenously once daily.
- If coadministered with cyclosporine, the recommended dose of PREVYMIS is 240 mg once daily.
- Please see full Prescribing Information for proper preparation and administration of PREVYMIS IV infusion.
Once-daily dosing
Dosage forms
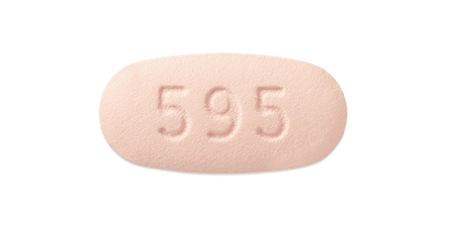
480 mg tablet
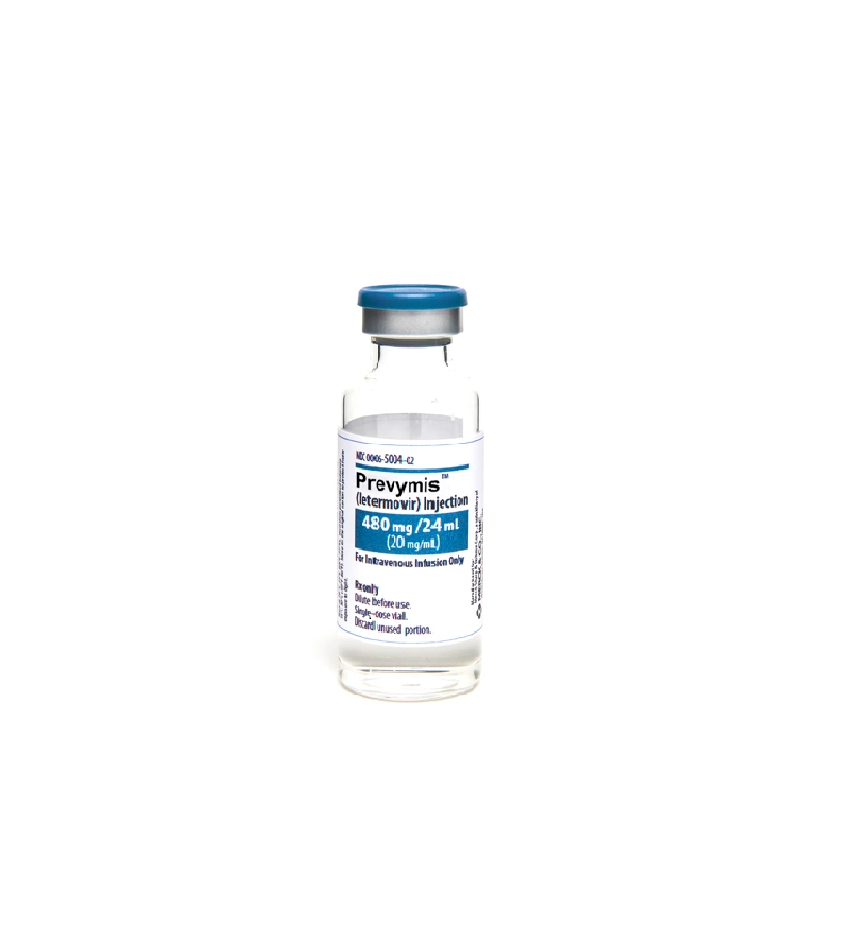
480 mg/24 mL vial

240 mg tablet
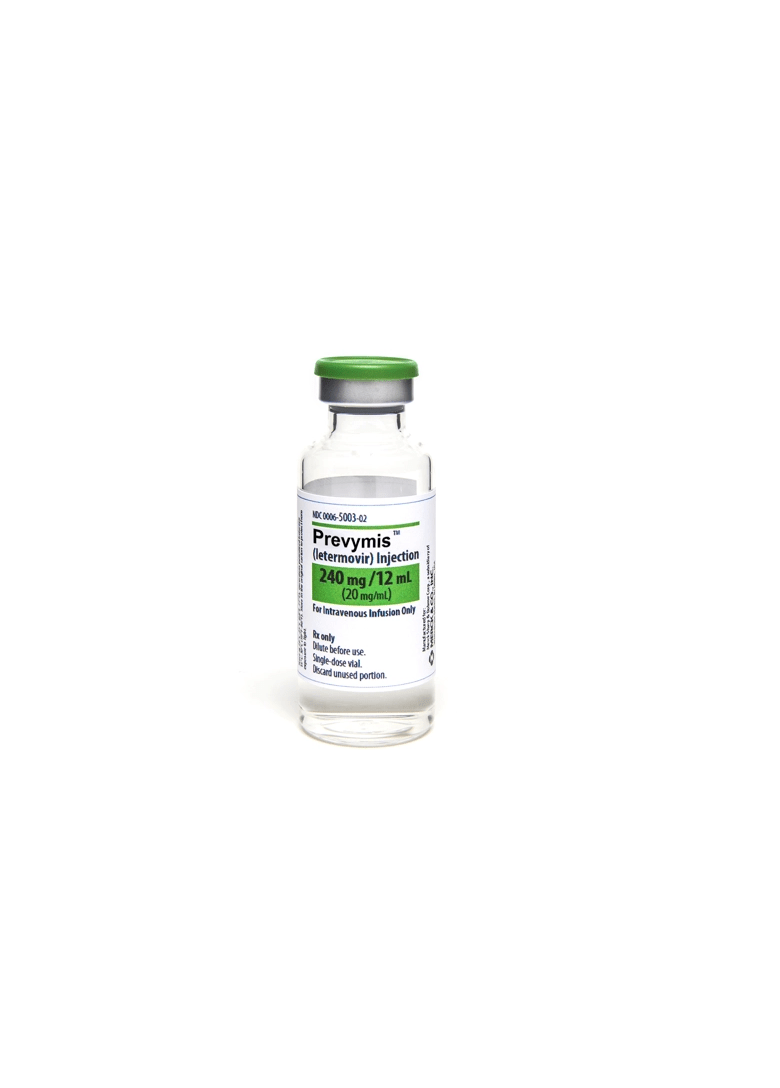
240 mg/12 mL vial
Tablets and vials not actual size.